Trust Transparency Center (TTC) has a history of bringing to light problems and issues within the dietary supplement that need to be addressed, or at least have some light shone on them. We have conducted product testing in several ingredient categories, including astaxanthin, coenzyme Q10, and curcumin. Through that testing, we have found—and called out—inconsistencies. One example: synthetic ingredients—in this case astaxanthin. Some companies claim, without scientific merit, to be "equivalent to natural" astaxanthin. We have commented on brand stories—transparent and otherwise.
Of course in the era of Internet marketing, barriers are lower than ever, making it even more difficult to detect "bad actors." Difficult, but not impossible. It does take effort, and as an example of the legwork that needs to be done, let’s take a case in point, and outline TTC's attempt to truly dig into the origins of one company that has been the subject of FDA scrutiny.
Wholesome Wellness is a supplement brand that claims to be headquartered in Portland, OR.
But where is it really?
From the Wholesome Wellness website FAQ page:
“Our headquarters are located in Portland, Oregon. We have a (sic) distribution centers across the USA. All of our products are manufactured in the USA in a GMP and FDA certified facility.”
Not that this is a red flag: There is no such designation as an “FDA Certified” dietary supplement manufacturing facility.
According to the label on the bottles, the address for Wholesome Wellness is listed as 6804 NE 79thCourt #527708 Portland, OR 97218.

TTC visited this address, and we saw no signage mentioning Wholesome Wellness. The building, located near Portland International Airport, appears to be a distribution warehouse. According to Tax Records, the address is registered to freight forwarder USGoBuy. While we were given the name of the owner of Wholesome Wellness, Jimmy Nguyen, no one we spoke with could tell us how to reach him.
The warehouse doors were open when we drove by, and we saw no sign of dietary supplements. Perhaps supplement were there, behind the lamps and boxes in the photo.

TTC tried reaching out to Wholesome Wellness customer support as the company website suggests, attempting to get more information regarding this operation. No response was provided. Consumers who bought the product online are asked to seek more information or advise the company of an adverse event. We tried to do so, and again, no reply was provided.
Another concern: Many companies seek to address/answer one-star reviews on Amazon with the reviewer in the dietary supplement industry, and Wholesome Wellness does not.
Amazon / FDA Anxiety and Stress!
As TTC has reported in the past, FDA and Amazon have taken efforts regarding greater product transparency. Somehow, though, Wholesome Wellness has seemed to find a way to skirt around both organizations’ requirements.
Amazon recently upped its standards to improve its practice and commitment to dietary supplement responsibility. While Amazon's programs are not complete and as thorough as we’d like to see in approaching the full Emerging Brand Scorecard (below), they have delisted suspect products reviewed byNOWand TTC testing reports. Amazon has also paid attention to FDA Warning Letters and delisted non-compliant products accordingly.
AnFDA Warning Lettersent to Wholesome Wellness earlier this year addressed the product “Natural Anxiety & Depression Relief.” The letter states that the Wholesome Wellness website was reviewed and that the agency noted several issues with the claims being made on the website. In an apparent response, Wholesome Wellness discontinued the product offering on its website, while continuing to sell on Amazon.
Our take on this: Wholesome Wellness discovered that the FDA does not scrub Amazon sites, and in fact, the company still uses some of the language complained about in the Warning Letter.
Yet another point of concern: the use of testimonials. Again, it appears to us that Amazon is lax in its policing, as evidenced by the content of the videos connected with Wholesome Wellness. One example:Anxiety Depression Relief Testimonial, transcribed for convenience for the reader:
“Hello, I was wanting to share with you guys this new Anxiety Depression Relief herbal supplement I found here on amazon. It has been great. I’ve been taking it for the last week. Um, here in this last year I was diagnosed with general anxiety disorder, which pretty much just means that I worry a lot and I worry about dumb things and my mind’s always racing and going, and I can get upset or worked up just about friends coming over to eat. And I really don’t feel comfortable taking the medication that they prescribe for those, like Ativan or Lorazepam. I don’t really want to take a narcotic, but I found these pills, the Anxiety Depression Relief and it’s a great herbal supplement. Has great things in it like St John’s Wort and Gingko Biloba. It’s just overall it has great things and for somebody looking to just ease their mind and find some kind of calm and rest, this really helps a lot. It helps you just wind down, calm down, makes your mind feel a little bit more at peace. I highly recommend trying these. They have been excellent.”
Product Efficacy
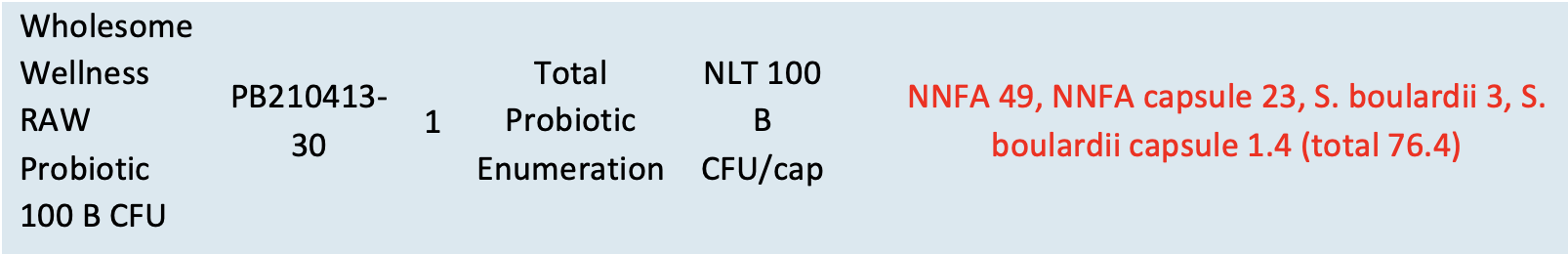
TTC had two of Wholesome Wellness’ products analyzed by multiple independent laboratories and found inconsistencies in ingredient content compared to label claim. (Probiotics and Digestive Enzymes were purchased after the FDA Warning Letter was issued.)
Digestive Enzymes with Probiotics:


There are 36 ingredients listed within the five label claim measurements provided. Still, by breaking out the expected specifications based on claim requirements, the test results indicate a failure in terms of total counts for probiotics and several individual components of ingredients.
Probiotics

The probiotic label claim is a minimum of 100B CFU/capsule. The test result from two different bottles purchased in June 2021 showing an enumeration total of only 76.4B CFU/capsule:
What’s the problem and where’s the true opportunity for responsible retailers and brands?
Individually, any of the issues discussed here pose a problem for the consumer:
- Not having a clear means of contacting a brand owner is an obvious problem because it does not afford a clear path for adverse event reporting.
- Using a freight forwarder address as a Corporate Address is deceptive.
- Using testimonial video to hide irresponsible marketing from the FDA is inexcusable.
- Using Amazon to hide a product flagged by the FDA is inexcusable.
- Creating and distributing product at less than label claim for the life of the product is inexcusable...and illegal.
Brick and mortar retailers have historically required demonstration of overall adherence to all regulatory requirements as a condition of retail placement. The barrier of entry into the dietary supplement market has decreased to the point that the allowance of brands challenged explicitly by the FDA is allowed to continue to exist. We as an industry are harmed when blatant irresponsibility is allowed to continue.
Trust Transparency Center has identified 17 significant areas for Regulatory Compliance in the form of a simple checklist for brands. This organization is in likely violation of most if not all:

Amazon has improved its quality and vetting procedures, but not enough to prevent these types of errors and omissions from occurring. It is important to note that thousands of dietary supplement companies, not just products, on Amazon, are not found on any other e-commerce platform. A bigger problem exists when a company chooses only to put a product on Amazon and not its direct website. We all have a role in order to improve the quality and reputation of the dietary supplement industry.
Note:WholeFoods Magazine reached out to Wholesome Wellness for comment and has not received a response. This article will be updated if a response is received.









