The name of this new supplement is a tough one to swallow, but swallowing the supplement brings many benefits. Geranylgeraniol (GG) is the name of this hidden compound that few have ever heard of. Let’s examine GG health benefits with Barrie Tan, Ph.D. We have discussed nutrients including tocotrienols in this column with Dr. Tan several times before, going back to 2008.
Dr. Tan earned his doctorate in chemistry with emphasis on biochemistry at the University of Otago, New Zealand. He later became a professor of chemistry and food science/nutrition at the University of Massachusetts, Amherst. His research expertise includes lipid-soluble materials such as carotenoids, tocotrienols/tocopherols, CoQ10, omega-3s and cholesterol. He has writtenThe Truth About Vitamin E: The Secret to Thriving with Annatto Tocotrienols, and was the lead editor of the second edition bookTocotrienols: Vitamin E Beyond Tocopherols.
Dr. Tan was the first to introduce the benefits of tocotrienols to the nutrition industry. He founded American River Nutrition in 1998, and developed the first-ever tocopherol-free tocotrienol product derived from annatto beans. Today, the focus of his research is on lipid-soluble nutrients that have an impact on chronic and degenerative conditions.
Passwater: Dr. Tan, ever since I met you some 35 years ago while you were still a professor at the University of Massachusetts, I have noticed your research interest in plant pigments, such as carotenoids, tocotrienols, and GG. You have more than 22 patents involving these nutrients (patents.justia.com/inventor/barrie-tan). Why do these nutrients attract your research interest?
Tan: I have always been intrigued by natural phenomena, and I love the nutrients both plants and animals make. Although we humans take many of our nature-derived products for granted, it’s important to realize that plants and mammals produce nutrients for specific purposes to promote their own physiological health and protection. I find great pleasure in exploring why nutrients are produced and how they function. Among my favorite compounds are lipid nutrients, which tend to stay around in the body for longer periods than water-soluble nutrients. These nutrients tend to be antioxidants and anti-inflammatories, and are known to mitigate chronic conditions. By building reserves within the body, they slow down the scourge of aging.
Passwater: Your patents on GG extraction go back to 2000. Dr. Tan, please give us an overview of what geranylgeraniol is, and why it’s so important?
Tan:I first learned about GG in the late 90s, coinciding with my work on vitamin E tocotrienols, which we began deriving from annatto at the time. We extract our tocotrienol product from the annatto plant, and our analyses showed that GG was also a component of the plant. But GG is not only a plant product; on the contrary, GG is a key molecule each human produces on a regular basis. In mammals, GG is a building block for the synthesis of CoQ10 and MK-4 (1), and it is required for the building of skeletal muscle proteins (2, 3).
Unfortunately, akin to CoQ10, GG synthesis drops as we age. A decline that may be associated with sarcopenia or age-related muscle loss. Further, a selection of pharmaceutical drugs is known to affect GG levels, most notably statins and bisphosphonates. Nearly 40 million Americans take statins, so this may affect a significant number of people.
Passwater:It’s interesting that GG is synthesized by both plants and mammals, but used in different ways. What role does GG play in the divergence of these two eukaryotes?
Tan:This is an evolutionary tale in biochemistry and nutrition. GG is a critical ingredient found in both plants and animals. This makes it unique, because few ingredients share such commonality. GG is the last common synthesis step of the mevalonate pathway between plants and mammals. In plants, GG originates terpenoids incorporated in curcuminoids, cannabinoids, menthol, thymol, and similar compounds. Color components called carotenoids, such as beta-carotene, lycopene, and lutein, as well as vitamin E (tocopherol and tocotrienol) and vitamin K1 (phylloquinone) are also derived from terpenoids. In mammals, GG originates isoprenoids producing coenzyme Q10, vitamin K2 (menaquinone-4), and squalene that leads to formation of cholesterol and sex hormones. These isoprenoids all originate from GG!
Here is an example of GG’s essential nature: GG is a precursor to chlorophyll that plants require for photosynthesis (to use carbon dioxide and exhale oxygen). Similarly, GG is a precursor to heme, which animals require for breathing (to use oxygen and exhale carbon dioxide). GG is indispensable to life itself.
Passwater: We have talked about vitamin E and tocotrienols in several of our previous interviews together. You mentioned that you derive tocotrienol and GG from the same plant source. Are the two molecules connected in other ways?
Tan: We obtain tocotrienol and GG from the annatto plant, a South American shrub that carries bright green or red pods filled with maroon seeds. As a carotenoid scientist, when I first encountered the annatto plant, I was astonished by the bright colors the plant radiated. A bright red stain coats anything touched by the oily seeds. At the time, annatto was solely used as a source of natural color for the food and cosmetics industry, with bixin being the plant’s primary carotenoid. I knew that the unstable bixin molecule must be protected from oxidation by a potent antioxidant, which we confirmed to be tocotrienol. When we analyzed the seed oil, however, we found not only tocotrienol, but also GG. When comparing the two compounds, the structures jumped out at me: GG was entirely fused into the tocotrienol molecule, making up its farnesyl tail. It became clear to me then that the plant uses GG to make tocotrienol (see Figure 1).
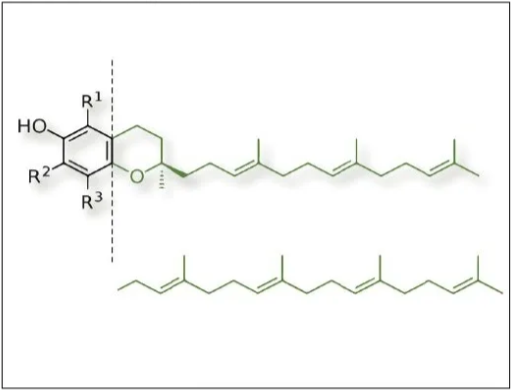
Ancient medicinal uses of annatto—its traditional use as a cardiotonic and anti-inflammatory agent—reflect what we know of GG and tocotrienol benefits today.
Passwater:Dr. Tan, can you please explain to us in greater detail how GG conveys its benefits in the human body?
Tan:GG is at the center of the mevalonate pathway, which is arguably the most trafficked pathway in our body. It is tasked with the synthesis of many important nutrients. Many know it as the cholesterol synthesis pathway, but it is a lot more diverse, producing many compounds critical to cellular function. As part of the isoprenoid pool that launches further biochemical reactions, GG is instrumental in the body’s production of CoQ10, MK-4, and skeletal muscle proteins (see Figure 2).
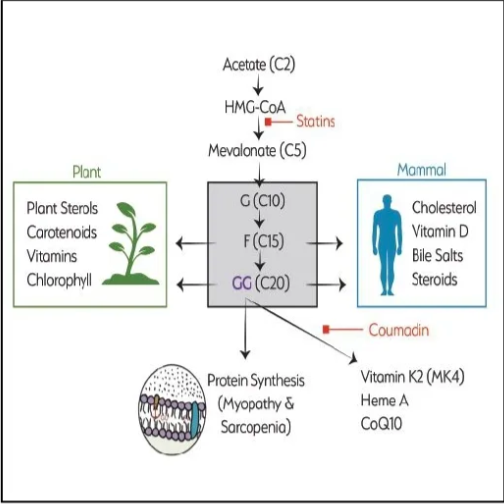
When it comes to energetics, GG is required for the biosynthesis of CoQ10 inside cells, in organelles where CoQ10 is a must-have component for energy production. The situation is similar for vitamin K2. MK-4 is the only menaquinone synthesized in our body. GG is a must-have component for endogenous MK-4 production. This again happens in the organelles, where MK-4 is used for enzymatic processing to transport calcium to bones while removing calcium from arteries.
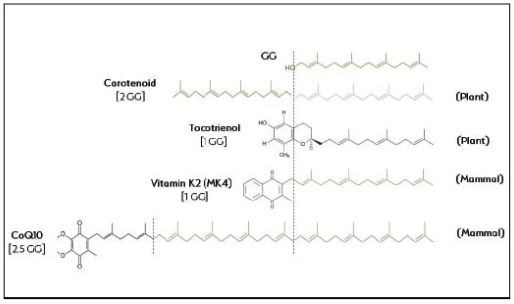
This GG-to-MK4 biosynthetic process is specific and GG is an exclusive nutrient for endogenous MK4 synthesis. GG’s role as a building block for other nutrients can be visualized when comparing molecular structures: One or more GG entities are directly inserted into molecules of CoQ10, MK-4, and carotenoids (see Figure 3 on page 37).
Further, GG is obligatory for the endogenous synthesis of skeletal muscle proteins. These proteins function as a scaffold or support structure for the shape and function we call muscles. A sizable 40% of our body weight is muscle, of which half is skeletal muscle protein. These require GG to regenerate muscle at all stages of life. In the young and energetic (~15-30yr old), GG helps increase force production. In mid-life (40-60yr), GG helps to maintain muscle mass relative to body weight. In the elderly (>65yr old) with progressive muscle mass loss (sarcopenia), GG helps to resist such loss, prevent muscle-associated atrophy, and increase thrive. This GG-to-muscle biosynthetic process is specifically for skeletal proteins. In animals, CoQ10, MK-4, and skeletal protein are ubiquitous because GG is ubiquitous.
Passwater:If you had an umbrella term to describe GG’s unique benefits, what would it be?
Tan:I would describe GG as a true “statin companion.” Statin is a universally successful medication for lipidemia treatment (particularly hypercholesterolemia) and has numerous pleiotropic benefits. However, statin is an indiscriminate cholesterol reducer in that it reduces other isoprenoid essentials along its path—including GG—besides cholesterol. Since these isoprenoids are far upstream, their impairment would manifest many downstream ramifications. Most notably, reduction of isoprenoids bears the crux of statin side effects. The nutrient most affected by statin damage is skeletal muscle protein, and secondarily CoQ10 (4).
CoQ10 is synthesized as an essential nutrient within our body to spark energy production. The fact that statin decimates CoQ10 production is well known (5), but it is less known that this effect on CoQ10 levels is fueled by the inhibition of GG, an intrinsic constitutional structure of CoQ10. For older individuals who already have lower CoQ10 (6), a combo with statin can have devastating effects on energy production. An astute individual who is on statin medications may supplement CoQ10 to remedy levels. Unfortunately, this is not nearly enough to address the root cause of statin side effects. GG is the “statin companion.”
Passwater:Your suggestion is that supplementing GG will replenish the isoprenoid pool, making more of the diterpene available for much-needed CoQ10 production in statin takers. Could you explain why statins affect skeletal muscle proteins, and how GG might help?
Tan:Certainly. The name for muscle problems caused by statins is SAMS—statin-associated muscle symptoms. About 40 million Americans are on statin medications and ~60% of patients reportedly experienced SAMS. It is by far the most reported statin side effects. Whereas 10-25% of SAMS were reported in independent observational studies, pharma-sponsored trials reported only <1% SAMS. It was explained during study randomizations that those who were unable to tolerate statins were excluded from trials during the washout period. Furthermore, SAMS problems were recorded only on the basis of creatine kinase (a muscle breakdown enzyme) being 10x above normal (7). This creates an implicit bias to the very low number of SAMS reported in these sponsored trials.
On the other spectrum, scientists and researchers disagree with objective measurements of SAMS (8, 9). It seems odd that after 35 years of a hugely successful “statin” drug, experts cannot come to terms with their most common muscle problem manifestation. The clinician-in-practice and statin patients are left to their own devices to manage this “inconvenient” problem.
Today, there is no medical intervention to treat SAMS. Statin’s enormous success to control run-away lipids has gained huge support from the American Heart Association (AHA), American College of Cardiology (ACC) and the European Society of Cardiology (ESC), and the European Atherosclerosis Society (EAS). These societies have promulgated guidelines for atherosclerosis and cardiovascular disease risk reduction through lipid management (10). The United States targets reduction of LDL levels of 70-189mg/dL by 30% and LDL levels of >190mg/dL by 50% (11).
Beyond reducing LDL, statins have also been recommended for use in the diabetic population, and it is one of the repurposed drug candidates to address COVID-19 infections (12).
All this is to say: Statins will remain the mainstay of lipid management, and their use will branch out into the elderly, diabetic, and potentially to COVID-19 populations. Consequently, manifestations of SAMS will be here to stay, and may possibly magnify. We desperately need a solution.
GG has been shown to reverse the side effects of statin drugs, specifically muscle atrophy. Statin-induced muscle damage was studied through the development of muscle atrophy. GG and farnesol are isoprenoids used in the cell membranes to “prenylate” or make proteins. Researchers first noted that atrogin-1 is the critical mediator of muscle damage (as in muscle fiber breakdown) induced by statin (13). Later the same group discovered that GG—but not farnesol—specifically prevented or rescued muscle damage caused by statins (14). Statin reduced myofiber by 60%, a state of muscular dysfunction that GG completely reversed. Furthermore, GG reduced statin-induced atrogin-1 by 65%. The researchers suggested that geranylgeranylation was required for the synthesis of the long tail of CoQ10. CoQ10 is an integral part of the mitochondrial respiratory complex that statin suppresses, causing mitochondrial dysfunction (15). The decrease in the latter may be responsible for muscle atrophy. Interestingly, both MK-4 and GG improved mitochondrial functions by increasing mitochondrial membrane potential, mitochondrial respiratory capacity, ATP production, and spare respiratory capacity. In so doing, MK-4 and GG both contributed to inhibit insulin resistance in the skeletal muscle (16). This statin-damage work was also compellingly verified in animal models (17). In muscles, statin increased atrogin-1, a characteristic of SAMS in rats, by about 100-150% followed by a consequent reduction of 30-35% muscle force production. GG supplementation clearly mitigated statin damage to muscle cells and increased force production to muscle.
Passwater:If GG reverses statin side effects, would it also counteract its cholesterol-lowering benefits?
Tan:This is an important question to answer, given that we want to take GG to prevent statin side effects, not its benefits. GG is not a substrate for cholesterol synthesis, but farnesol is. A brief look at the mevalonate synthesis pathway can clarify this mechanism (see Figure 4). GG should not affect statin’s ability to lower cholesterol because cholesterol is synthesized directly from farnesyl pyrophosphate, and GG cannot be converted to farnesyl pyrophosphate or cholesterol. Earlier animal studies actually suggest that GG may have a cholesterol-lowering effect (18).
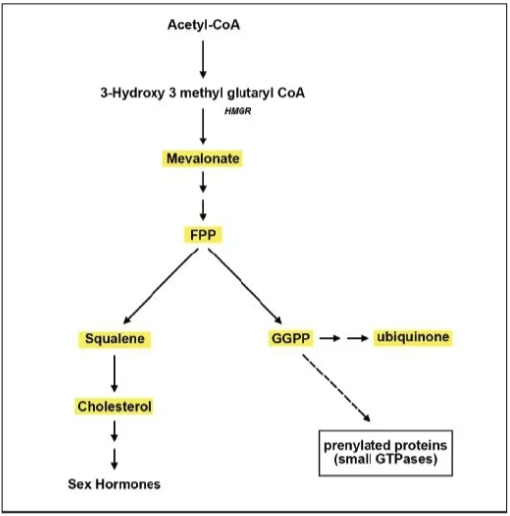
Hence, GG does not interfere with statin benefits, but instead restores ubiquinone and skeletal muscle protein production.
Passwater:Aside from preventing statin-associated muscle symptoms, does GG improve muscle function?
Tan:GG’s role in skeletal muscle production doesn’t just apply to deficiency states such as those induced by drugs. Instead, one of GG’s most basic tasks includes the making of proteins via a process known as prenylation. In a nutshell, GG attaches to a protein like a prosthetic arm, thereby enabling the protein to be made while attached to membranes. With 40% of the body’s weight being mostly skeletal muscle that requires isoprenoid prenylation, GG plays a vital role in keeping us strong.
One animal study examined the effects of GG—using a human equivalent dose of 170mg/day—on exertion muscles in young, healthy rats (19). GG was able to avert skeletal muscle fatigue and increased muscle force production, suggesting enhanced exercise performance. On a vascular level, GG increased relaxation in muscular arteries, which may be connected to enhanced nitric oxide when GG was supplemented.
Beyond exercise performance, GG has important implications for age-related loss of muscle mass. Known as sarcopenia, the progressive skeletal muscle disorder affects approximately 10-40% of the aging population and results not only in a greater risk of falls and fractures, but also muscle atrophy. Atrogin-1 is a gene indicated in the development of muscle atrophy, and GG was found to reduce atrogin-1, thereby preventing muscle fiber damage (14).
Passwater:You mentioned that GG is a building block not just for protein synthesis, but also other important endogenous nutrients, such as CoQ10. Please explain in more detail.
Tan:As an intermediate in ubiquinone synthesis, two and a half GG molecules make up the length of the CoQ10 tail. Radiolabeling studies have confirmed GG’s role as an intermediate in CoQ10 synthesis, where the radiolabeled GG becomes part of the CoQ10 molecule (1). Moreover, GG is required for the prenylation of CoQ10 in the Golgi apparatus, a process that is further catalyzed by a protein-encoding gene called UBIAD1 (20). While increasing CoQ10 levels, GG aids in the restoration of mitochondrial functioning (16, 21).
Similar to CoQ10, MK-4 contains a number of isoprenoid side chains linked together, where GG is obligatory in the synthesis of this menaquinone (20, 22-24). This has also been confirmed through radiolabeling (1). MK-4 is the most abundant menaquinone in the body, and through its integral connection with GG, can tackle important maintenance jobs to deter aging, including calcium transport and bone mineralization.
GG’s indelible link to MK-4 may yet contribute to an additional bodily function: Both molecules were able to stimulate testosterone production (25, 26). One study in testis-derived cells showed that GG could regulate the steroidogenesis pathway to increase both testosterone and progesterone levels. The effect on hormone regulation was achieved through protein kinase A, an important biological messenger signal the body uses (26). This aging-in-place GG will help the elderly to thrive with normalized levels of testosterone.
Passwater:Other than GG’s ability to prevent statin drug side effects, are there any other potential drug interactions?
Tan:There is another class of drugs that specifically interacts with the mevalonate pathway. These drugs are called bisphosphonates, and they are generally used in the treatment of osteoporosis to slow bone loss. Nitrogen-containing bisphosphonates, in particular, can bind specifically to bone cells and block the isoprenoid farnesol, hence interfering with protein prenylation. While this helps preserve bone in most of the body, a lack of bone turnover and capillary maintenance areas of the body with higher remodeling rates can have severe implications. However, bisphosphonates are known to cause osteonecrosis along with altered wound healing and chronic infections, a condition known as bisphosphonate-related osteonecrosis of the jaw (BRONJ) (27). A lot is studied and written on this subject.
Fortunately, GG can mitigate the adverse effects of bisphosphonates. In several studies, GG was able to reverse the deleterious effects of bisphosphonates by decreasing apoptosis of the longest-living bone cells called osteocytes (28), as well as increasing osteoblast differentiation and angiogenesis (29, 30). Further safeguarding the bone in the jaw, GG also preserved mesenchymal stem cells, which are important differentiator cells for bone building (31), and inhibited the formation of bone-dissolving osteoclasts (32).
Those with BRONJ typically have significant problems with wound healing, especially following tooth extractions, and are prone to ulcers, reopening of wounds, and necrosis of the bone (33). In BRONJ animal models, GG consistently improved bone healing of extraction sockets and prevented osteonecrosis, while also warding off infection and decreasing inflammation scores (28, 34).
Passwater:The research is fascinating and certainly anticipates a promising future for GG. As a never-before-seen and brand-new product, are there any safety concerns with GG?
Tan:We have painstakingly taken our time to launch GG and to ensure that the product meets all quality and regulatory standards. As such, GG is self-affirmed GRAS, and we have completed rigorous toxicological studies according to OECD guidelines (35). Based on the GRAS evaluation, GG can be used in foods and beverages such as nutrition bars and sports drinks at servings up to 250mg, while the toxicological assessment found GG to be safe at dosages up to ~400mg per day.
Passwater:That’s the maximum dose. How about the most effective doses, and in what manner should GG be taken?
Tan:Based on human equivalence calculations from animal studies, as low as 70mg/day of GG will affect testosterone levels, while 150mg per day for general use or as a statin companion product is appropriate. As a plant-based lipid oil, GG should be taken with a meal.
Passwater:Would GG and CoQ10 complement each other?
Tan:Absolutely! As we discussed, GG can help increase CoQ10 levels, and when GG and CoQ10 are supplemented together, they can provide a science-driven synergistic cardiovascular support platform. While both supplements boost mitochondrial efficiency, GG adds the unique benefits for enhanced muscle function. In addition to our GG-Gold product, we are currently working on a unique combination product called DuoQuinol, which combines GG and CoQ10 in a proprietary process to yield ubiquinol.
Passwater:Has there been any clinical research with GG?
Tan:American River Nutrition is the first company to bring GG to the market, and we are also the first to conduct clinical trials with this endogenous nutrient. Currently, we are actively recruiting for a placebo-controlled sexual health study for both men and women. The study has a unique dose escalation design, where subjects will receive 150mg of GG daily for the first 4 weeks, and 300mg per day for an additional 4 weeks. In the process, we will keep track of testosterone, estrogen and progesterone levels, as well as sex hormone binding globulin and safety panels.
In another double-blind, placebo-controlled, randomized intervention trial, conducted at Texas Tech University Health Sciences Center, we are recruiting 75 subjects that are taking statin drugs and have muscle-related side effects. Participants will receive a placebo, 150mg, or 300mg GG for a period of 12 weeks, and will be tested for muscle performance, muscle damage, and inflammation. Muscle pain levels will be surveyed via questionnaires throughout the study.
Further studies are planned to evaluate GG’s effect on exercise endurance and other outcomes.
Passwater:Very interesting research! What are some closing thoughts you would like our readers to take home?
Tan:It’s important to remember that GG is a universally present, endogenous nutrient—it is made naturally by our body and is a building block for essential-to-life nutrients. CoQ10 is ubiquitous because GG is ubiquitous, and MK-4 is ubiquitous because GG is ubiquitous. Like CoQ10, GG decreases as we age, and is also negatively affected by widely used drugs such as statins or bisphosphonates. This is where supplementation helps! For the first time, GG is available as a supplement, GG-Gold, derived naturally from the annatto plant. The ingredient is currently available as an oil, powder, and bulk softgel.
With statin taking the number-one spot of most prescribed drug in the U.S., and associated muscle side effects the most common occurrence, GG is an obvious statin companion product.
New scientific findings are on the horizon for this emergent “compound of life,” and we look forward to sharing forthcoming clinical trial results over the next few years.
Passwater:This is a new supplement and thus is not yet widely available. Where can readers find product information for geranylgeraniol?
Tan: American River Nutrition offers GG-Gold as a bulk ingredient, but our partner finished product listings can be found on our website atwww.americanrivernutrition.com.
Passwater: Thank you once again Dr. Tan for developing an important nutritional supplement and for explaining its biochemistry. WF
Note: The views and opinions expressed here are those of the author (s) and contributor (s) and do not necessarily reflect those of the publisher and editors of WholeFoods Magazine. Information in this interview is intended for educational and scientific purposes only. It is not intended as medical or nutritional advice for the treatment or prevention of disease. For medical advice, consult your personal health care practitioner.
References- Stoffel, W. and C. Martius, Zur Synthese der K-Vitamine und Ubichinone. Angew Chem, 1960. 72(17): p. 627-627.
- Crick, D.C., D.A. Andres, and C.J. Waechter, Novel salvage pathway utilizing farnesol and geranylgeraniol for protein isoprenylation. Biochem Biophys Res Commun, 1997. 237(3): p. 483-7.
- Crick, D.C., C.J. Waechter, and D.A. Andres, Utilization of geranylgeraniol for protein isoprenylation in C6 glial cells. Biochem Biophys Res Commun, 1994. 205(1): p. 955-61.
- Mollazadeh, H., et al., Effects of statins on mitochondrial pathways. J Cachexia Sarcopenia Muscle, 2021. 12(2): p. 237-251.
- Päivä, H., et al., High-dose statins and skeletal muscle metabolism in humans: A randomized, controlled trial. Clinical Pharmacology & Therapeutics, 2005. 78(1): p. 60-68.
- Kalén, A., E.-L. Appelkvist, and G. Dallner, Age-related changes in the lipid compositions of rat and human tissues. Lipids, 1989. 24(7): p. 579-584.
- Pedersen, T.R., et al., High-Dose Atorvastatin vs Usual-Dose Simvastatin for Secondary Prevention After Myocardial InfarctionThe IDEAL Study: A Randomized Controlled Trial. JAMA, 2005. 294(19): p. 2437-2445.
- Taylor, B.A. and P.D. Thompson, Statin-Associated Muscle Disease: Advances in Diagnosis and Management. Neurotherapeutics, 2018. 15(4): p. 1006-1017.
- Thompson, P.D., et al., Statin-Associated Side Effects. J Am Coll Cardiol, 2016. 67(20): p. 2395-2410.
- Feldman, D.I., et al., Same evidence, varying viewpoints: Three questions illustrating important differences between United States and European cholesterol guideline recommendations. Am J Prev Cardiol, 2020. 4: p. 100117.
- Grundy, S.M., et al., 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 2019. 139(25): p. E1082-e1143.
- Memel, Z.N., et al., Association of Statins and 28-Day Mortality Rates in Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J Infect Dis, 2022. 225(1): p. 19-29.
- Hanai, J., et al., The muscle-specific ubiquitin ligase atrogin-1/MAFbx mediates statin-induced muscle toxicity. J Clin Invest, 2007. 117(12): p. 3940-51.
- Cao, P., et al., Statin-induced muscle damage and atrogin-1 induction is the result of a geranylgeranylation defect. FASEB J, 2009. 23(9): p. 2844-54.
- Apostolopoulou, M., A. Corsini, and M. Roden, The role of mitochondria in statin-induced myopathy. Eur J Clin Invest, 2015. 45(7): p. 745-54.
- Su, X., et al., Vitamin K2 Alleviates Insulin Resistance in Skeletal Muscle by Improving Mitochondrial Function Via SIRT1 Signaling. Antioxid Redox Signal, 2021. 34(2): p. 99-117.
- Goodman, C.A., et al., Statin-Induced Increases in Atrophy Gene Expression Occur Independently of Changes in PGC1alpha Protein and Mitochondrial Content. PLoS One, 2015. 10(5): p. E0128398.
- de Moura Espindola, R., et al., Geranylgeraniol and beta-ionone inhibit hepatic preneoplastic lesions, cell proliferation, total plasma cholesterol and DNA damage during the initial phases of hepatocarcinogenesis, but only the former inhibits NF-kappaB activation. Carcinogenesis, 2005. 26(6): p. 1091-9.
- Irwin, J.C., et al., Validation of a clinically-relevant rodent model of statin-associated muscle symptoms for use in pharmacological studies. Toxicol Appl Pharmacol, 2018. 360: p. 78-87.
- Hirota, Y., et al., Functional characterization of the vitamin K2 biosynthetic enzyme UBIAD1. PLoS One, 2015. 10(4): p. E0125737.
- Campia, I., et al., Geranylgeraniol prevents the cytotoxic effects of mevastatin in THP-1 cells, without decreasing the beneficial effects on cholesterol synthesis. Br J Pharmacol, 2009. 158(7): p. 1777-86.
- Harshman, S.G., et al., Atorvastatin Decreases Renal Menaquinone-4 Formation in C57BL/6 Male Mice. J Nutr, 2019. 149(3): p. 416-421.
- Huang, H., et al., Structure of a membrane-embedded prenyltransferase homologous to UBIAD1. PLoS Biol, 2014. 12(7): p. E1001911.
- Schumacher, M.M., et al., Geranylgeranyl-regulated transport of the prenyltransferase UBIAD1 between membranes of the ER and Golgi. J Lipid Res, 2016. 57(7): p. 1286-99.
- Ho, H.J., et al., A novel function of geranylgeraniol in regulating testosterone production. Biosci Biotechnol Biochem, 2018. 82(6): p. 956-962.
- Ho, H.J., et al., Geranylgeraniol enhances testosterone production via the cAMP/protein kinase A pathway in testis-derived I-10 tumor cells. Biosci Biotechnol Biochem, 2016. 80(4): p. 791-7.
- Gupta, M. and N. Gupta, Bisphosphonate Related Jaw Osteonecrosis, in StatPearls. 2022: Treasure Island (FL).
- Chen, X., et al., Geranylgeraniol Restores Zoledronic Acid-Induced Efferocytosis Inhibition in Bisphosphonate-Related Osteonecrosis of the Jaw. Front Cell Dev Biol, 2021. 9: p. 770899.
- Mungpayabarn, H. and S. Patntirapong, Timing of geranylgeraniol addition increases osteoblast activities under alendronate condition. J Oral Biol Craniofac Res, 2021. 11(3): p. 396-401.
- Zafar, S., et al., Effects of zoledronic acid and geranylgeraniol on angiogenic gene expression in primary human osteoclasts. J Oral Sci, 2020. 62(1): p. 79-83.
- Singhatanadgit, W., W. Hankamolsiri, and W. Janvikul, Geranylgeraniol prevents zoledronic acid-mediated reduction of viable mesenchymal stem cells via induction of Rho-dependent YAP activation. R Soc Open Sci, 2021. 8(6): p. 202066.
- Hiruma, Y., et al., Vitamin K2 and geranylgeraniol, its side chain component, inhibited osteoclast formation in a different manner. Biochem Biophys Res Commun, 2004. 314(1): p. 24-30.
- Politis, C., et al., Wound Healing Problems in the Mouth. Front Physiol, 2016. 7: p. 507.
- Koneski, F., et al., In vivo effects of geranylgeraniol on the development of bisphosphonate-related osteonecrosis of the jaws. J Craniomaxillofac Surg, 2018. 46(2): p. 230-236.
- Preece, K., et al., A toxicological evaluation of geranylgeraniol. Regul Toxicol Pharmacol, 2021. 124: p. 104975.










