This column recently covered the Amazon flywheel, and its application to the natural retail space. The concept of the flywheel is a means to generate power. The flywheel is constructed of a small wheel that generates power to exponentially power a larger wheel resulting in greater power overall.
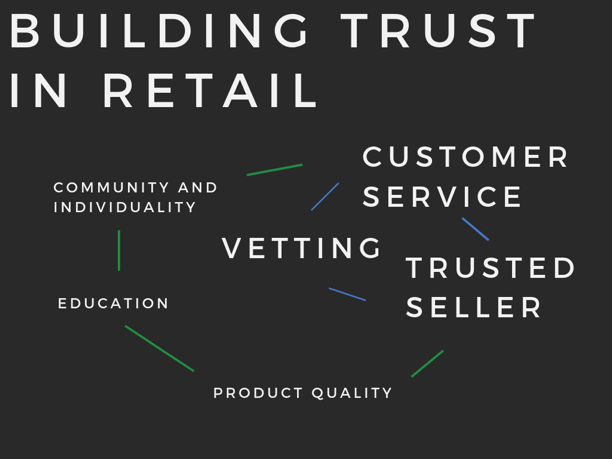
The core suggested key components for a natural retail store flywheel are:
- Customer Service
- Trusted Seller
- Product Quality
- Education
- Community and Individuality
Currently, Amazon has 16,227 brands of dietary supplements listed on the US Amazon.com site. The brand listing results in 245,001 associated SKUs. To sell dietary supplements on Amazon, an organization simply completes an application that “requires” the seller to comply with all laws and regulations in the territory the product will be sold. It does not require an affirmation that the seller knows what those laws and regulations are or proof of meeting the same. It’s a passive system at best.
The checklist system ‘appears’ to have been a barrier to some products. Case in point: There are currently 1,391 products listed on Amazon’s prohibited supplements list, but for most, there is no apparent rhyme or reason as to why the products are listed as prohibited. Amazon merely states that they didn’t meet the checklist requirements. There are some big brands on this list, such as:
- Walmart “Spring Valley” Echinacea
- Walmart “Spring Valley” Garlic
- Walmart “Spring Valley” Ginseng
- GNC "Herbal Plus" Echinacea
- GNC "Herbal Plus" Ginseng
- Walgreens "Finest Nutrition" Echinacea
- Walgreens "Finest Nutrition" Ginseng
- CBD (Cannabidiol)
Amazon also has a post-market system in which it will delist products. There are a variety of transgressions for which this can occur, and a mechanism for reinstatement, but again the system is rather opaque.
It is clear the opportunity to vet suppliers is a key differentiator for a natural retailer competing with Amazon. There are many questions that can be asked to determine the quality and legitimacy of some brands. Transparency of information is the starting point, so a “no” answer to any vetting questions can be a non-starter to accepting a product into your store – a locked “gate” if you will.
Here are 10 basic vetting questions retailers should ask brands:
- What’s the history of the company (founded when/by whom/credentials)?
-
Where is the product manufactured and how are the manufacturers qualified?
- Some brands are manufactured “in-house” in a wholly owned manufacturing facility.
- Most brands are manufactured by contract manufacturers. Many brands will not list specific contract manufacturers, but they should be willing to share a complete list of them without being product specific. Transparency of manufacturing is becoming an increasingly important criteria for consumers.
- All manufacturers should be cGMP compliant and willing to demonstrate their registrations and certificates of inspection/quality.
- How are ingredients sourced/tested?
- How and when is stability testing conducted?
- Who is responsible for brand quality control and what are their qualifications for the role?
- What is the lot tracking process?
- What is the expiration dating process?
- What 3rd party testing processes are in place?
- Are allergen statements available from suppliers to support each product?
- How are adverse events reported, recorded and handled? Is there a recall plan in place?