Rich Passwater, Jr., my elder son, has been involved with silicon supplementation and its research for more than 16 years. He is the education director for the Belgium-based dietary supplement research and dietary supplement production company, Bio Minerals NV.
Richard A. Passwater (RAP): Hair relies on the protein keratin for strength and thickness. Is silicon important to keratin production?

Richard Passwater, Jr. (RPJr): Yes, silicon in the form of biologically active orthosilicic acid (OSA) is important both directly by helping the body make and strengthen keratin and indirectly through its critical roles in collagen and elastin production.
The hair formation site, called the dermal papilla, is a collagen-based tissue at the bottom of hair follicles. A dermal papilla looks like an old-fashioned incandescent light bulb or like a fist. The cells on the outer layer of the derma papilla are filled with keratin fibers. Once filled, these cells are pushed away from the dermal papilla and become incorporated into the hair shaft. So, hair “grows” one layer of keratin fibers after another, pushed out and away from the dermal papilla.
The dermal papilla generally gets smaller as people grow older and lose collagen. As a result, the hair becomes thinner because the surface area around the dermal papilla that gets filled with keratin becomes smaller.
If you can keep collagen production high by returning it to more youthful levels, the dermal papilla can return more to the size it was when a person was younger. The bigger the dermal papilla, the thicker and stronger the hair fiber.
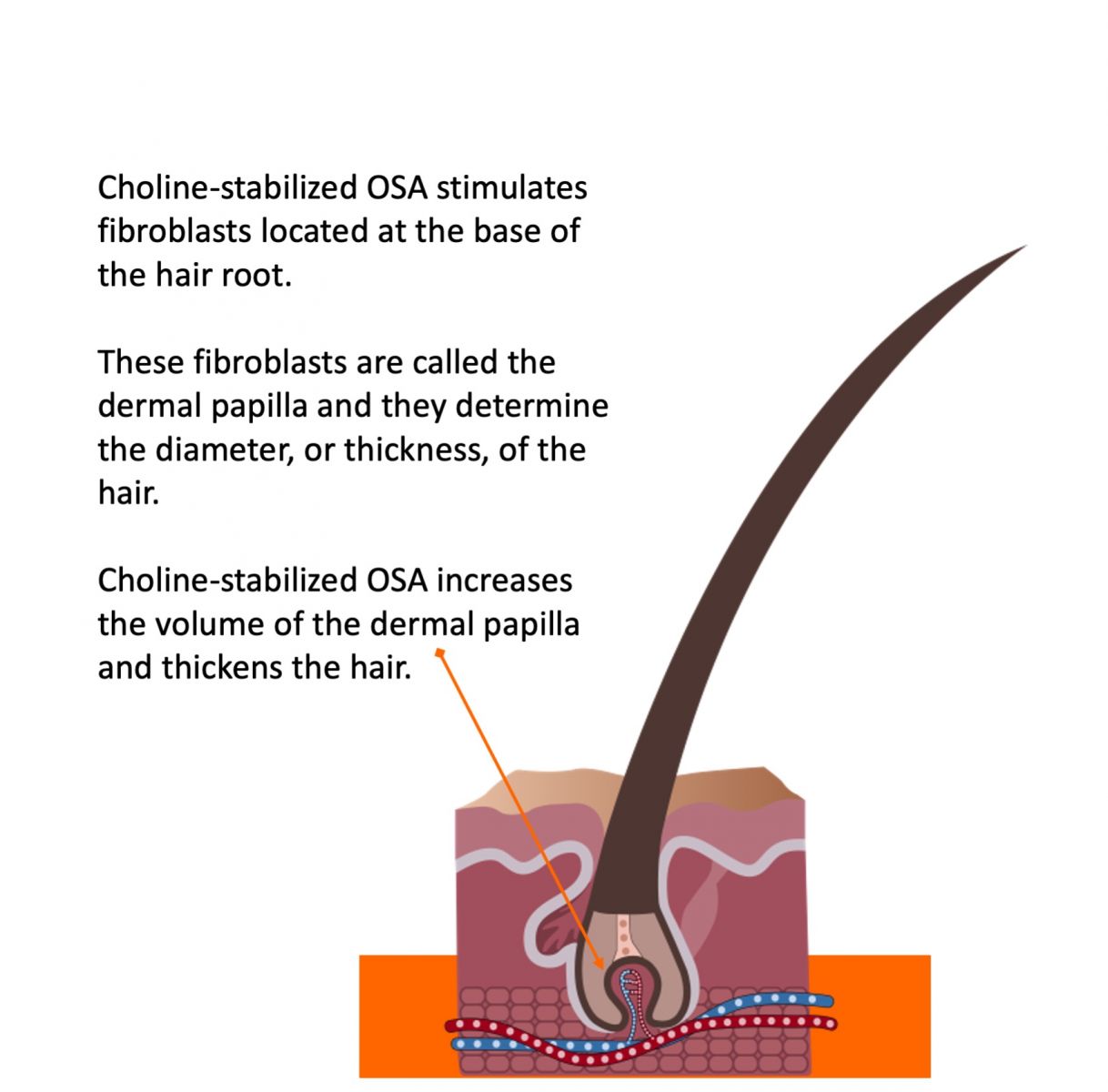
Secondly, blood flow to the dermal papilla is critical for both the rate (or speed) of hair growth and the quality of hair fibers.
Increasing collagen and elastin production in the skin and in the body’s 60,000 miles of blood vessels improves microcirculation. This results in better blood flow, and better nutrient delivery, to the dermal papilla. Better circulation generally results in healthier, faster-growing hair.
Thirdly, the enzyme, ornithine aminotransferase, is silicon dependent. This enzyme makes l-proline, an amino acid that makes up about 6-9% of keratin, the protein making up about 97% of hair.
Fourthly, silicon is incorporated directly into the hair structure to improve the strength of the keratin by linking the keratin fibers together.
RAP:Nails are somewhat similar to hair; is silicon important to nails too?
RPJr: Yes. Nails and hair are very similar, and silicon is involved the same way.
One difference is the nail formation site is called the nail matrix. Like the dermal papilla, the nail matrix generally gets smaller as people age. And as with hair, blood flow and enzymatic activity in the nail matrix are also reduced with age. But these factors do not have to occur if you can keep collagen and elastic production high.
Another difference between hair and nails is that nail structures can be influenced by diet and lifestyle as long as the nail plate is attached to the skin, whereas hair is pretty much biologically dead tissue once it grows away from the dermal papilla.
For this reason, people often notice changes to their nails first when making diet and lifestyle changes.
RAP: Generally, the amount of anything that someone has in their body is the net between how much they produce and how much they lose. We have discussed collagen production. What are some of the causes of collagen loss?
RPJr: In my opinion, four of the leading causes of collagen damage throughout the body are (1) homocysteine, (2) stress hormones such as cortisol, (3) free radical damage, and (4) sugar, first through the glycation reaction and then from advanced glycation end products (AGEs).
Homocysteine is an amino acid continuously being produced by the body as part of normal methionine metabolism. Methionine is an essential amino acid and the body has a very ingenious recycling system for it. In some of the methionine metabolism reactions, the methionine is converted into homocysteine. Then it is converted back into methionine or cysteine using nutrients.
Unfortunately, as people age, the conversion of homocysteine into methionine or cysteine generally decreases; often due to the lack of the nutrients required to make the conversion. This results in elevated homocysteine levels.
Also, it is very common for homocysteine levels to rise, and even spike, after menopause. One reason is the estrogen dependent enzyme PEMT (phosphatidylethanolamineN-methyltransferase) activity decreases. PEMT helps the body make choline, one of the nutrients capable of converting homocysteine back into methionine. But the PEMT enzyme requires estrogen to function properly. So postmenopausal women don’t have the same potential to make choline endogenously as premenopausal women and thus face more challenges relative to managing homocysteine levels.
Accumulation of homocysteine has a negative impact on collagen throughout the body. Homocysteine can be called “the anti-collagen amino acid” because it directly damages existing collagen and suppresses new collagen production.
In particular, the accumulation of homocysteine has a negative impact on collagen several ways. First, homocysteine reacts chemically with collagen, interfering with collagen cross- linking. Also, homocysteine thiolactone can be produced from homocysteine and it directly inhibits the activity of a critical collagen generating enzyme, lysyl oxidase. Furthermore, homocysteine also interferes with collagen production by down-regulating the expression of lysyl oxidase’s messenger RNA and other genes involved in beneficial collagen cross-linking.
High homocysteine is probably most known as a risk factor for stroke. And for a good reason—it damages the collagen and elastin making up about 40% of the structure of blood vessels.
High homocysteine levels damage collagen everywhere in the body. As an example, high homocysteine levels correlate with higher hip fracture incidence and low Bone Mineral Density (BMD).
It is common for women to lose 30% of their collagen in the five years following menopause. As mentioned above, it is common for homocysteine to spike during and after menopause.
Part of this collagen loss comes from a decrease in collagen production as estrogen levels decrease. Estrogen has a positive effect on the rate of collagen and elastin production. But some of the 30% collagen loss is related to increased homocysteine levels destroying existing collagen and suppressing collagen production. The homocysteine-related damage does not have to happen. The loss can be prevented with proper nutrition.
It is well documented that nutrients such as folic acid, vitamin B12, choline and its metabolite betaine convert damaging homocysteine back into beneficial methionine. Vitamin B6 can convert homocysteine into beneficial cysteine.
For these reasons, folic acid, vitamin B12, choline, betaine, and B6 protect collagen matrixes throughout the body.
Cortisol, much like homocysteine, both destroys existing collagen and suppresses collagen production by interfering with lysyl oxidase activity and most likely other collagen- generating enzymes. So, cortisol is like a one-two punch for collagen destruction.
I think a perfect model to observe cortisol’s impact on skin collagen is to look at how most presidents show rapid aging. They often show significant signs of aging after just four years. The damage is evident in their skin, as it reflects less light and gets thinner, and you can often see dark circles under the eyes.
Cortisol’s negative effects occur throughout the body. As an example, a group of stress researchers in the UK conducted a test where they injected rats with cortisol to observe its impact on connective tissue. Those researchers observed that cortisol damaged skin collagen at 10 times the rate that it damaged bone collagen. Some of those researchers speculated that perhaps the body prioritizes collagen content in different tissues and may value, protect, and maintain some collagen tissues more than others.
Lack of sleep also leads to an increase in the stress hormone cortisol, and a decrease in growth hormones that are necessary for collagen production. Corticosteroids, such as drugs for asthma or autoimmune diseases, or cortisone shots, can all increase cortisol levels and lead to collagen loss.
Sugar is a commonly overlooked destroyer of collagen throughout the body. Non-enzymatic reactions that covalently attach sugar molecules to a protein or lipid (fat) are called glycation. Glycation can occur through Amadori reactions, Schiff base reactions, and Maillard reactions, which lead to advanced glycation end products (AGEs). Sugar reacts negatively with proteins, especially proteins high in the amino acid lysine, a significant building block of collagen. This makes collagen very susceptible to glycation damage. Glycation is a big part of the reason diabetics often look much older—sometimes even 15% older—than they are.
AGES are collagen and elastin destroyers. Some AGES are formed inside the body as secondary reactions after a glycation reaction. Others are introduced to the body from the diet or environment. Eating charred food is often overlooked as a source of introducing AGES into the body. AGES often damage collagen networks without someone’s knowledge.
Sun exposure is the biggest radical generator associated with skin collagen damage. But environmental pollutants and especially smoking can also damage and reduce the functionality of collagen fibers and even fibroblasts, thus causing significant collagen problems. Perhaps smoking is the number one thing people can do to damage collagen. It not only damages collagen but it uses up copious amounts of vitamin C, which can further suppress new collagen production.
RAP: More than 15 years ago, it was reported that silicon’s systemic absorption of bioactive silicon as choline-stabilized OSA produced rapid results actually observable and measurable by reduced skin wrinkling as well as internal health benefits (Barel et al. 2005). These measurable indicators also include improved nail and hair strength. This clinical study shows that in just 20 weeks, shallow wrinkles improved by 30% and skin elasticity measurements improved by 89%, as well as a significant reduction of brittleness in nails and hair compared to the placebo group.
RPJr: That Barelet al. study is an impressive one. To my knowledge, it is the first randomized, double-blind, placebo-control intervention study published in a peer-reviewed medical journal documenting a nutrient-complex making statistically significant and clinically relevant improvements to skin, hair, and nail quality.
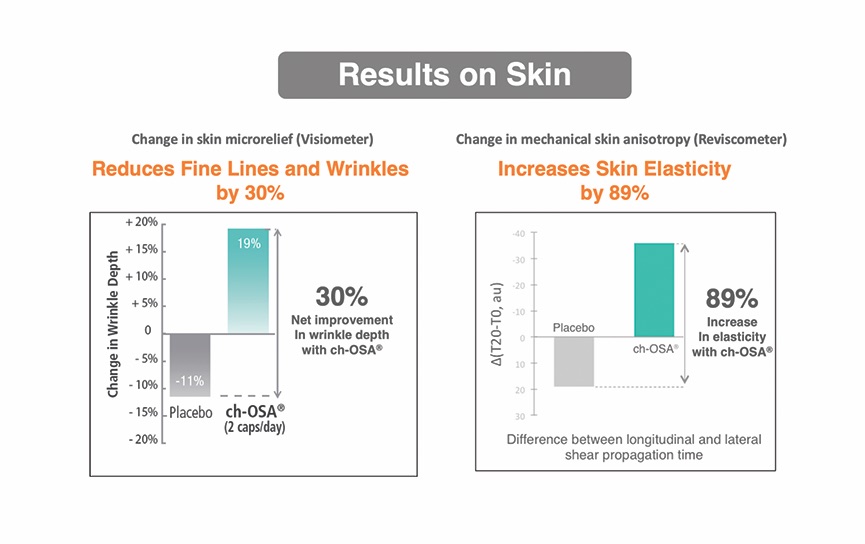
Women between 40 and 65 years old took either one choline-stabilized OSA capsule (BioSil) twice daily or one placebo capsule (containing plant fiber) twice daily for 20 weeks.
The wrinkle depth and skin elasticity improvements the women taking choline-stabilized OSA experienced are related to improvements in the quantity and/or quality of collagen and elastin in their skin.
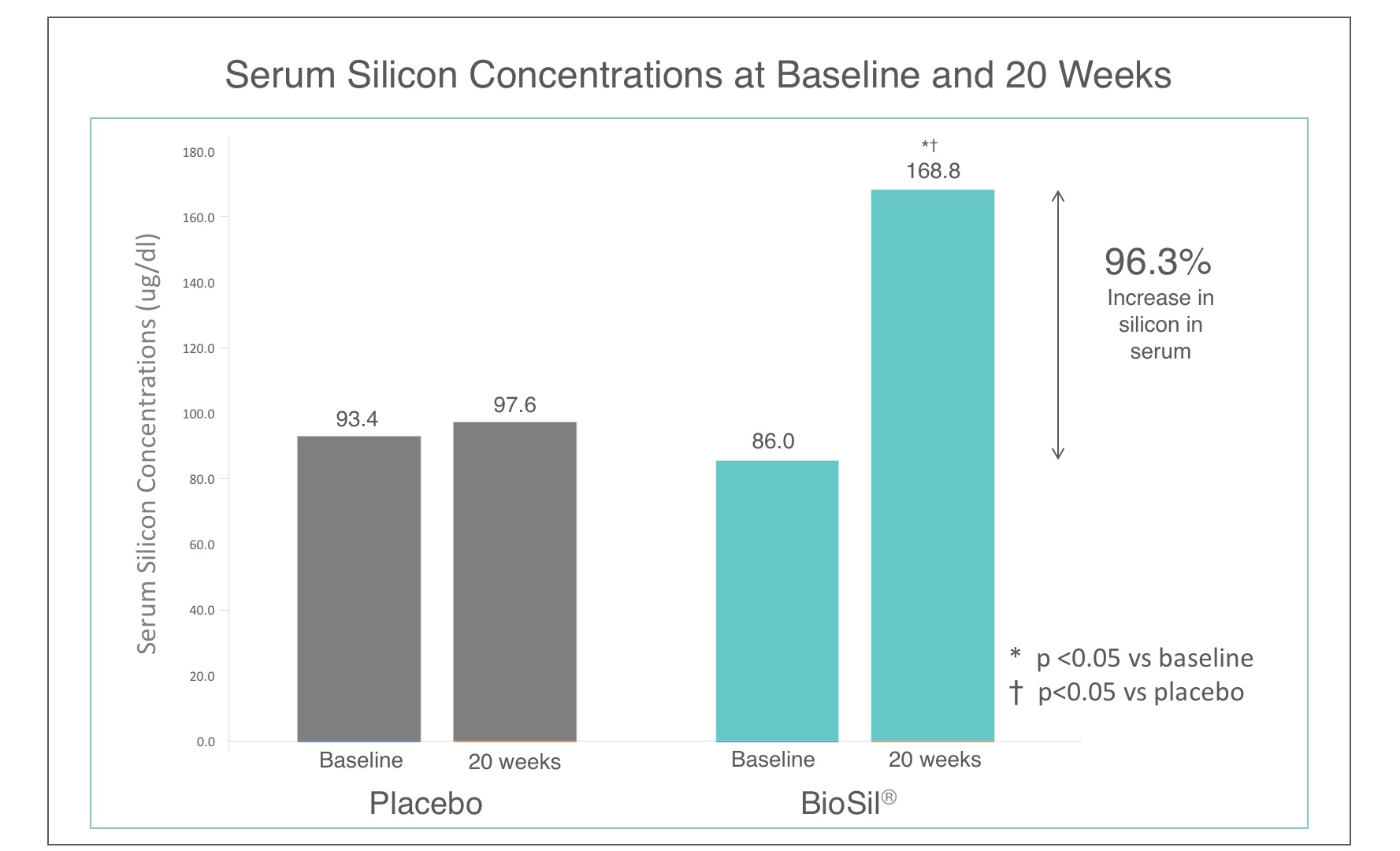
At the time of the study, BioSil’s source of OSA became the star. Adding a modest, 10 mg per day dose of elemental silicon in the form of choline-stabilized orthosilicic acid to a standard diet, generally containing about 20-50 mg of elemental silicon per day, increased serum silicon concentrations by over 96%.
As we discussed earlier, OSA is an essential cofactor for the activation of the collagen-generating enzymes ornithine aminotransferase, prolyl hydroxylase and lysyl hydroxylase. Ornithine aminotransferase and prolyl hydroxylase are also critical enzymes involved with producing elastin.
Choline-stabilized OSA contains silicon but is much more than an orthosilicic acid delivery system. Choline-stabilized OSA is a unique complex. Some of the benefits are from silicon, some from choline, and some from the choline-stabilized OSA complex itself.
Some examples of the benefits from OSA:
- Activating key enzymes required for collagen generation such as ornithine aminotransferase, prolyl and lysyl hydroxylases.
- Performing other structure linking roles to strengthen collagen fibers and keratin fibers.
- Neutralization of homocysteine to protect collagen and protect lysyl oxidase activity.
- Emerging research also suggests choline can help regulate the stress hormone, cortisol.
- Also, choline has roles in modulating inflammation.
- Choline helps the body produce l-glycine, the most prevalent amino acid in collagen.
- Preventing OSA molecules from reacting with each other or proteins while in the bloodstream.
- Allowing higher concentrations of OSA than otherwise possible.
- Assisting in transporting OSA into collagen generating cells.
In addition, recently, emerging research suggests choline can help regulate levels of collagen-destroying cortisol. Controlling cortisol levels can also prevent collagen and elastin damage. It’s very possible that cortisol modulation might have something to do with the results.
Today, I firmly believe the improvements observed in this study were not only related to OSA increasing collagen and elastin production but also by choline-stabilized OSA protecting the volunteers’ existing and new collagen and elastin from being damaged by homocysteine and cortisol.
RAP: How about clinical studies on OSA and hair?
RPJr:Much of the hair research studying choline-stabilized OSA has focused on hair thickness and strength. As an example, in a 9-month study (Wickett, 2007) women with fine hair taking two choline-stabilized OSA capsules (BioSil) per day increased their hair strength about 13.1% and their hair thickness about 12.8% compared to women taking placebo pills.
RAP: What about clinical studies of choline-stabilized OSA on nails?
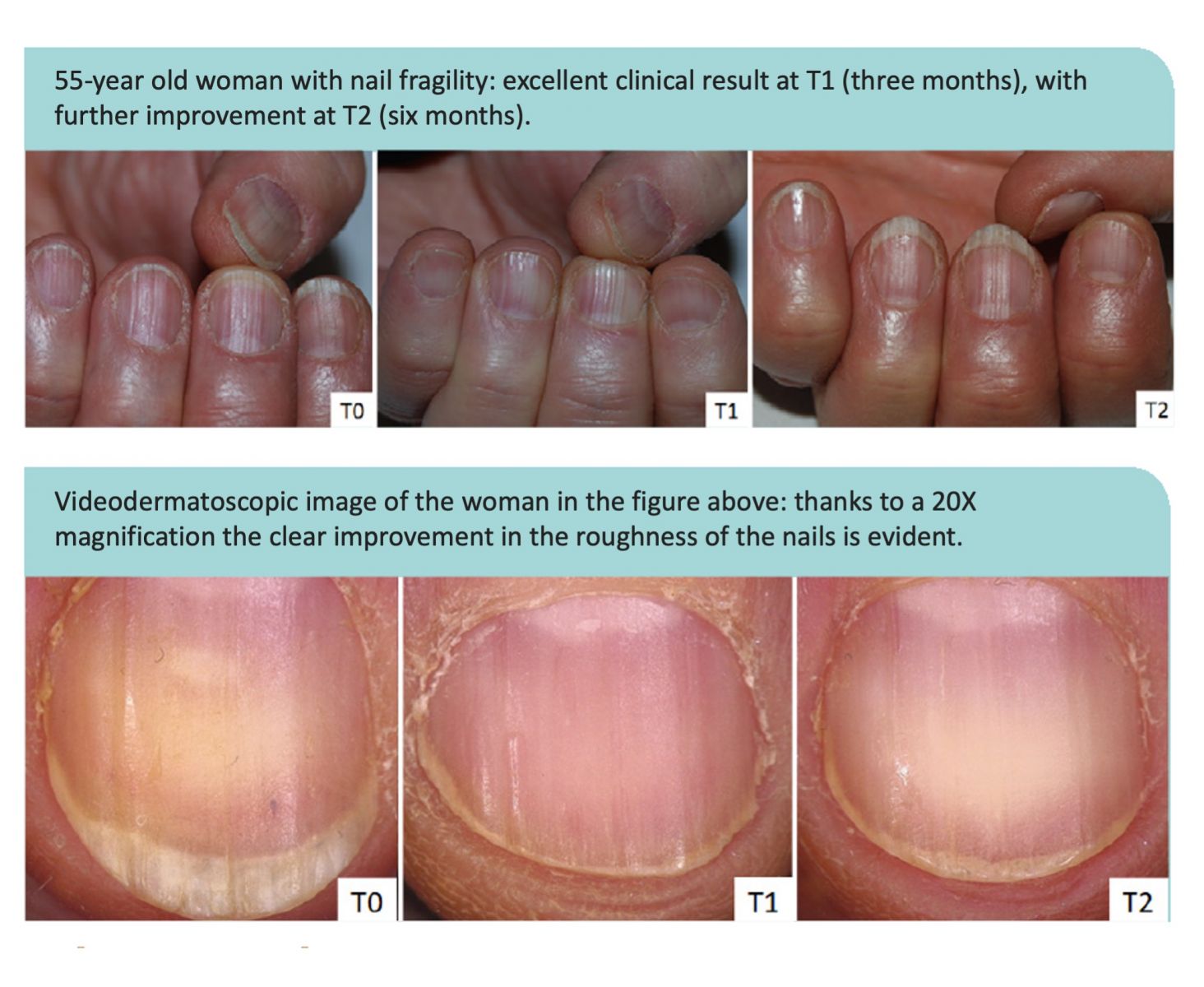
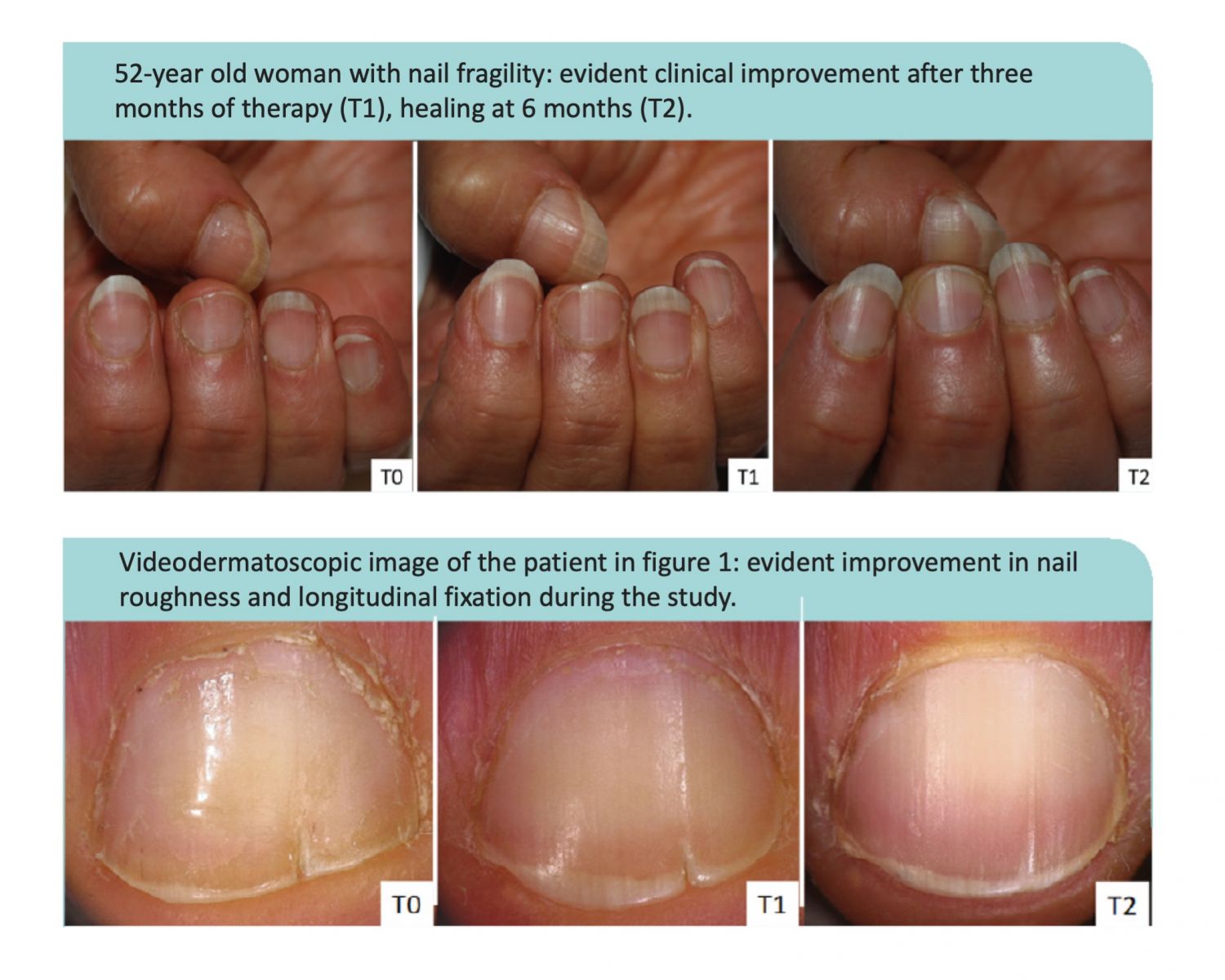
RPJr: The most recent clinical study investigating choline-stabilized OSA’s impact on nail health was presented at the 93 Annual Italian Dermatology Society (SIDeMaST) Congress in Bologna during May 2018. A group of researchers from the University of Bologna Medical School’s Dermatology department presented a study on women (average age 59.2 years old, range 52-65) who had rough, brittle nails. Brittle nails are a common problem experienced by about 20% of the general population and over 40% of some subgroups such as postmenopausal women.
70% of the women in the Bologna study also had vertical nail ridges and 30% also had problems with splitting of the nail plate. In this 6-month study, the principal investigator concluded 100% of the women taking two choline-stabilized OSA capsules (BioSil) per day reduced the roughness and brittleness of their nails and 83% reduced the vertical ridges. Evaluations made by additional investigators were made using a 20x magnification system. The researchers felt all the women taking BioSil improved the quality of their nails; determining 44% “completely improved” and 56% “much improved.”
Examples from the study are pictured below.
 RAP: Please review some of the research on bones.
RAP: Please review some of the research on bones.RPJr: A human clinical study performed at the University of London’s St. Thomas Hospital and published inBMC Musculoskeletal Disorders is a great example. In this study, a group of women recently diagnosed with osteopenia were all given 1,000 mg of calcium and 800 IU of vitamin D3.
People with osteopenia have a DEXA scan T-score between -1 and -2.5. A healthy person around 35 years old should have a T-score of 0 and a person with a T-score more negative than -2.5 is diagnosed with osteoporosis, a disease condition where the risk of fracture is greatly elevated. So, osteopenia is sort of the midpoint between having healthy bones and having osteoporosis.
Some women took a placebo while others took choline-stabilized OSA (BioSil). The study demonstrated that adding choline-stabilized OSA to a standard calcium and vitamin D supplement regimen increased the production of bone collagen by 15% (measured as procollagen type I N-terminal propeptide) and Bone Mineral Density (BMD) at the critical hip region by 2% compared to calcium and vitamin D supplements alone.
I know, 2% might not sound like a lot. But the standard for clinical relevance—or a meaningful difference—is a 1% increase after three years versus a placebo. And in the choline-stabilized OSA study, the researchers observed twice this threshold in just one year, compared to calcium and vitamin D3.

choline-stabilized OSA (BioSil) improves hair, skin and nails.
In my opinion, the hip is the most important area to focus on in bone research because hip fractures are often the deadliest and debilitating. As an example, about one in three adults aged 50 and over dies within 12 months of suffering a hip fracture. Also, only about 30% of elderly people who survive a hip fracture can return to their previous level of independence. Hip fractures are really a leading cause of death and debilitation in the western world.
RAP: Thanks for sharing your knowledge with our readers. At times, we have been very technical in our discussion, which I felt was necessary for many readers. However, there are faithful and deserving readers who only want the practical advice without all of the theory and explanations. I have found the 2015 book, “Timeless Beauty,” by supermodel Christie Brinkley (Pub. Grand Central Life & Style), to provide excellent practical unsolicited advice on using choline-stabilized OSA (BioSil) for skin, hair and nails.










