Editor’s Note: This article is intended for information purposes only. Because state and municipal laws vary greatly, as do the circumstances of individual cases, readers are advised to contact an attorney for specific legal advice. The views and opinions expressed here are those of the author(s) and contributor(s) and do not necessarily reflect those of the publisher and editors of WholeFoods Magazine.
Throughout the year 2023, the U.S. Food and Drug Administration (FDA) has had over-the-counter (OTC) ophthalmic products in its crosshairs. In April 2023, and after an 11-day inspection that had begun in February, the FDA found that the Global Pharma Healthcare facility in India did not follow proper protocol to prevent bacterial contamination of its eye products. The company recalled its Artificial Tears Lubricant Eye Drops, distributed by EzriCare and Delsam Pharma, even before the FDA’s inspection was completed. Yet, the Centers for Disease Control and Prevention (CDC) reported four deaths and 14 instances of eye loss after their use (1).
Then, on August 22, 2023, the FDA released its warning to “consumers not to purchase and to immediately stop using Dr. Berne’s MSM Drops 5% Solution and LightEyez MSM Eye Drops – Eye Repair due to bacterial contamination, fungal contamination, or both” (2). The FDA, however, admitted that there were no adverse events associated with the products (3).
Not yet finished, on September 11, 2023, the FDA then sent warning letters to eight other companies, six of which were homeopathic companies, claiming that the companies’ eye drops constituted “an unapproved new drug under section 505(a) of the Federal Food, Drug, and Cosmetic Act (FD&C Act), 21 U.S.C. 355(a)” and that “no FDA-approved application … is in effect” (4).
The eight recipients of these FDA Warning Letters were Boiron Inc., CVS Health, DR Vitamin Solutions, Natural Ophthalmics, Inc., OcluMed LLC, Similasan AG, TRP Company, Inc., and Walgreens Boots Alliance, Inc. Although the FDA alleged CGMP violations in several of the letters, again there were no adverse events reported for any of these products.
On November 13, 2023, Kilitch Healthcare India Limited announced a voluntary recall of 27 different eye-drops products that it manufactures for Rite Aid, Leader, Target, Velocity, and other retail outlets. The announcement stated that “these products are being recalled due to potential safety concerns after FDA investigators found insanitary [sic] conditions.” (emphasis added)
While some homeopathic products are being swept up in the general FDA multi-year dragnet of OTC eye drops, I must agree with Alvin Lorman, general counsel for the American Association of Homeopathic Pharmacists (AAHP), when he wrote that, “it would be premature to consider this action part of an FDA targeting homeopathy” (5).
The Anti-Homeopathy Agenda
And yet it is curious that this recent FDA action against homeopathic eye drops is taking place against a general backdrop of anti-homeopathic propaganda. The FDA itself helpfully warns on its website “Do not use ophthalmic products that: … [a]re labeled as homeopathic, as these products should not be marketed” (6). This seems like a rather stark and ill-informed opinion coming from a Federal agency actually created by a homeopath.
Then, for more fun, just Google search “American Association of Homeopathic Pharmacists statement on eye drops” and, yes, you will see as the first result the AAHP’s statement, but following that will be nearly countless links such as “Every homeopathic eye drop should be pulled off the market” (Slashdot), “Every homeopathic eye drop should be pulled off the market” (Ars Technica), “Stop using homeopathic eye drops” (Popular Science), “Eyedrops from CVS, Rite Aid and others carry possible infection risk, FDA says” (PBS), and “What’s Driving Patients Towards Homeopathy?” (Review of Ophthalmology). The knives are all out, it seems, for homeopathy (7).
So Where Are All the Bodies?
The AAHP was determined to find out what the true facts about the risks of homeopathy might be after the FDA cited 10,311 reported adverse events related to “homeopathic agents” in the 2012 American Association of Poison Control Centers’ Annual Report (AAPCC). An “analysis showed that the number of exposures to homeopathic medicines in any given year is less than one percent of all pharmaceutical reports to AAPCC, which is proportionally below the rate of the market share for homeopathic medicines” (8).
Further analysis was done, and statistics compiled for homeopathic agents from the 2012–2019 Annual Reports of the American Association of Poison Control Centers’ National Poison Data System (NPDS), which can be seen in the following chart from the AAHP website (9):
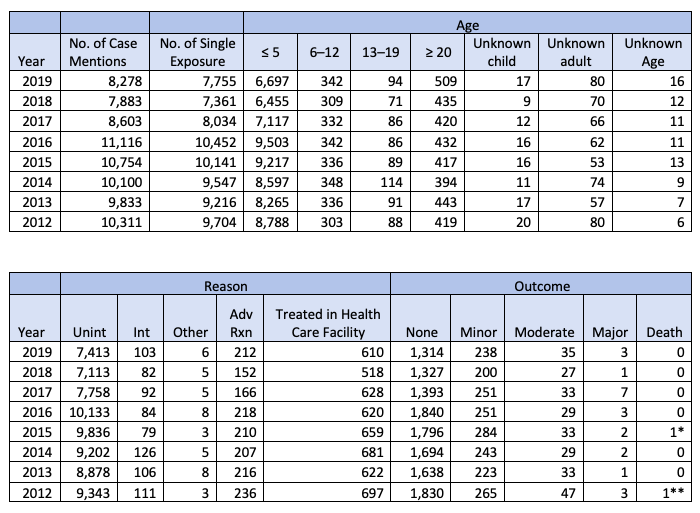
During the eight years covered in the chart, there were only two probable deaths from homeopathic agents (as they like to call them). But even those two deaths come under a category of “Dietary Supplements/Herbals/Homeopathic,” with the first death involving the use of yohimbe, and the second “supplement, botanical” and cocaine (10). That’s hardly conclusive evidence for even one death genuinely attributable to a homeopathic remedy.
And as for the other adverse events’ outcomes, the vast majority of them are classified as minor and moderate. The few major adverse events are in the exceptionally low single digits. In all, this is an incredibly impressive safety record for homeopathic remedies, a record that allopathic drugs have never achieved.
Interestingly enough, COVID-19 introduced an increase in ocular complaints, which homeopathy could easily and safely address, such as dry eye, conjunctivitis, and other such conditions. As noted in a 2021 meta-analysis, one in ten people with COVID-19 experienced ocular issues (11). The allopathic pharmaceutical industry might have more ocular drugs planned and in the pipeline, with a new post-COVID-19 market emerging and the competition needing to be eliminated.
FDA-Approved Drugs Are More Dangerous
The FDA wants to impose upon homeopathy an allopathic regulatory regime using the same “risk-based” enforcement approach that has brought consumers such disasters as Vioxx (60,000-500,000 deaths), Avandia (83,000+ deaths), Ibuprofen (17,000 deaths/year) (12), and chemotherapy (9 million since year 2000) (13). That’s right, we haven’t had any deaths from homeopathic products in 30 years, so we really need to make sure it catches up with modern medicine and its hundred-thousand-deaths-per-year sterling record under the FDA’s watchful regulatory eye (14).
The FDA is supposed to protect the lives of American citizens; what it actually does is protect the grotesque profits of the big pharmaceutical companies, at the expense of American lives, in a complete abuse of its mission. In fact, if you put “FDA corrupt” into an online search engine, you will be swamped with examples.
The FDA aspires to a “risk-based approach” to enforcing consumer health and well-being. But as it worries itself sick about the chipped paint on the windowsill, the entire house is burning down to the ground. In fact, the FDA Commissioner Robert Califf himself has reported that in the first nine months of the year 2023, there have been 158,000 more deaths in America than occurred in 2019 (15). While some pretend to scratch their heads as to the reasons behind this dramatic increase in deaths, especially among young adults, others, such as Dr. Pierre Kory, lay the blame squarely at the feet of the FDA, which granted emergency use authorizations to largely untested, experimental injections mislabeled as “vaccines” to increase their acceptance by the public. This unfolding disaster may very well be the greatest example of FDA gross misconduct or crime ever.
If the FDA genuinely wants to prioritize its enforcement activities, then there is no better way to do that than to unleash the goon squads on the allopathic pharmaceutical industry that is killing one-hundred thousand Americans a year with its dangerous vaccines and drugs alone (16). The bodies pile up in great heaps around that industry. But where are the bodies of those who take homeopathic remedies? Do I really need to point out the obvious: that the FDA cannot prove even a single death definitively caused by homeopathy?
Instead, we have a growing number of children who are born healthy, get over-vaccinated, and then are never healthy again. It is a public-health crisis that can no longer be ignored and yet the FDA is ignoring it. Rather than chase after minor injuries among homeopathic patients, the FDA needs to tend to the real health dangers to our children: vaccine injuries that kill and maim tens thousands of children every year (17). This is where the bodies are. This is the real health problem that needs immediate attention, not homeopathy. The most ironic part about the FDA’s supposed quest for child safety is that its suppression of homeopathy will deprive us of the very tools that could help heal the vaccine injured, among many others (18).
Has the FDA Ever Gotten Anything Right?
Supporters of the FDA often turn to the thalidomide-baby tragedy and the FDA’s handling of that drug recall as a premiere example of FDA efficacy in action. And, yet, as health writers and scientists Durk Pearson and Sandy Shaw pointed out decades ago, the FDA actually caused more harm than good by its actions. Thalidomide was a medication given in the late 1950s and early 1960s to pregnant women to ease their morning sickness and other complaints. Unfortunately, it also caused birth deformities in babies born to mothers on that drug. When the FDA removed it from the market, its place was taken by highly addictive barbiturates, which actually led to an increase in deaths from accidental overdoses. Both Marilyn Monroe and Judy Garland were two celebrities who died of barbiturate overdoses. A simple black-box warning on thalidomide packaging and a prohibition against its use by pregnant women would have avoided many of these deaths from barbiturates.
From personal observations of the international scene, and after having attended numerous Codex Alimentarius meetings during the last 24 years, I can safely say that I have rarely, if ever, observed the FDA taking a correct position on any health standard or guideline at Codex. There is an extreme disconnect between the American people and their government representatives, the latter of whom push every single anti-health and pro-industry measure at Codex meetings that one could possibly imagine. If it is anti-health, then the U.S. Codex delegates are right there, championing its adoption. This includes opposing GMO labeling, pushing ractopamine and zilpaterol doping of animals for human consumption, pushing a “sky’s the limit” exemption for melamine poisoning of infant formula, backing higher levels of aluminum in our foods, lowering therapeutic values in dietary supplements, and the list goes on.
The Way Out for Homeopathy
On March 27, 2015, the FDA published a Federal Register announcement that it would hold a public hearing to evaluate the regulatory framework for homeopathic products. This led to more than six years of effort by the FDA to revise its guidance on homeopathic remedies, creating much uncertainty in the industry and finally resulting in no real positive regulatory outcome. The FDA is clearly incapable of creating a regulatory framework for the homeopathic industry.
Experienced food-and-drug attorney Todd Harrison, a partner with the Washington, D.C. law firm Venable LLP, said it best when he observed, “We live and die by product enforcement. The FDA can easily remove homeopathic products if they want. What we need is an established pathway to market for homeopathic drugs that is not part of the allopathic drug-approval pathway. This may require a legislative change to the regulatory framework for the marketing of homeopathic drugs according to homeopathic principles and not allopathic principles” (19). I agree. WF
References
- Carma Hassan, “Another Death, More Cases of Vision Loss Linked to Contaminated Eye Drops, CDC Reports,” CNN, May 19, 2023, at https://www.cnn.com/2023/05/19/health/ezricare-eye-drops-recall-update/index.html.
- FDA News Release, “FDA warns consumers not to purchase or use certain methylsulfonylmethane (MSM) eye drops due to contamination,” FDA, Aug 22, 2023, updated Aug 30, 2023, at https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-consumers-not-purchase-or-use-certain-methylsulfonylmethane-msm-eye-drops-due.
-
Ibid.
-
See, e.g., FDA Warning Letter to Boiron Inc., Sept 11, 2023, at https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/boiron-inc-663402-09112023; and FDA Warning Letter to Walgreens Boots Alliance, Inc., Sept 11, 2023, at https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/walgreens-boots-alliance-inc-663404-09112023.
-
Alvin J. Lorman, “Increased FDA Scrutiny of Eye Care Category,” theAAHP.org, Sept 18, 2023, at https://theaahp.org/regulatory/increased-fda-scrutiny-of-eye-care-category/.
-
FDA Consumer Information, “What You Should Know about Eye Drops,” FDA, updated Dec 12, 2023, at https://www.fda.gov/drugs/buying-using-medicine-safely/what-you-should-know-about-eye-drops.
-
See, e.g., Ian Musgrave, “No Evidence Homeopathy Is Effective: NHMRC Review,“ The Conversation, April 8, 2014, at https://theconversation.com/no-evidence-homeopathy-is-effective-nhmrc-review-25368.
-
AAHP staff writer, “Poison Control Centers Statistics on Homeopathy,” AAHP, Jan 11, 2021, at https://theaahp.org/compliance/poison-control-center-statistics-on-homeopathy/.
-
Ibid.
-
Ibid.
-
Nasiri N, Sharifi H, Bazrafshan A, et al., “Ocular Manifestations of COVID-19: A Systematic Review and Meta-analysis,” Journal of Ophthalmic & Vision Research, Jan-Mar 2021, 16(1): 103-112, at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7841281/.
-
Alexander Cockburn, “When Half a Million Americans Died and Nobody Noticed,” The Week, April 27, 2012, at http://www.theweek.co.uk/us/46535/when-half-million-americans-died-and-nobody-noticed.
-
Scott Tips, “Restore Integrity to the FDA, Remove the Pharmaceutical-Industry Infiltrators,” Whole Foods Magazine, March 2017, at https://wholefoodsmagazine.com/columns/legal-tips/restore-integrity-fda-remove-pharmaceutical-industry-infiltrators/.
According to one source, 290 Americans are killed by prescription drugs each and every day. See Starfield B, the Journal of the American Medical Association, Vol. 284, No. 4, Johns Hopkins School of Hygiene and Public Health (2000). Yet another one says that 2,460 Americans die per week from prescription drugs. See Michael Schroeder, “Death By Prescription,” U.S. News, Sept 27, 2016, at https://health.usnews.com/health-news/patient-advice/articles/2016-09-27/the-danger-in-taking-prescribed-medications. Others, still, have pegged the death count at almost ten times the first number.
-
Sandra Rose, “FDA Commissioner Sounds the Alarm on ‘Catastrophic’ Rise in Death Rate Since 2019,” SandraRose.com, Dec 14, 2023, at https://sandrarose.com/2023/12/fda-commissioner-sounds-the-alarm-on-catastrophic-rise-in-death-rate-since-2019/.
See Lucian Leape, “Error in Medicine,” Journal of the American Medical Association, 1994, 272(23): 1851-1857, at p. 1851; Lucian Leape, “Institute of Medicine Medical Error Figures Are Not Exaggerated,” JAMA, July 5, 2000, 284(1): 95-97, doi:10.1001/jama.284.1.95.
- Robert F. Kennedy, Jr., "Hiding Vaccine-Related Deaths With Semantic Sleight-of-Hand," GreenMedInfo, July 18, 2017, at https://greenmedinfo.com/blog/hiding-vaccine-related-deaths-semantic-sleight-hand.
-
Kate Birch, “Vaccine Free: Homeopathic Education for the Immune System,” VaccineFree, undated, at https://vaccinefree.wordpress.com/vaccine-injury-homeopathic-detox-mn-cease/.
-
Conversation with Todd Harrison, Esq. on December 19, 2023. Harrison’s comments here were not intended in any way to be an endorsement of the author’s views but were general observations from his decades of legal experience.










